NanoScience Lab
Physics at the nanoscale
Nanostructured materials (NMs) represent an active area of research and a techno-economic sector with increasing expansion in many application domains. The technological importance of NMs is due to their tunable physicochemical characteristics such as tunable optical absorption, electrical and thermal conductivity, chemical state and bactericidal functionalities. The possibility of tailoring the properties of NMs is an open matter that may push their use into a variety of innovative fields.
Below a short description of the main current research topics
Tailoring nanoparticles by gas phase synthesis
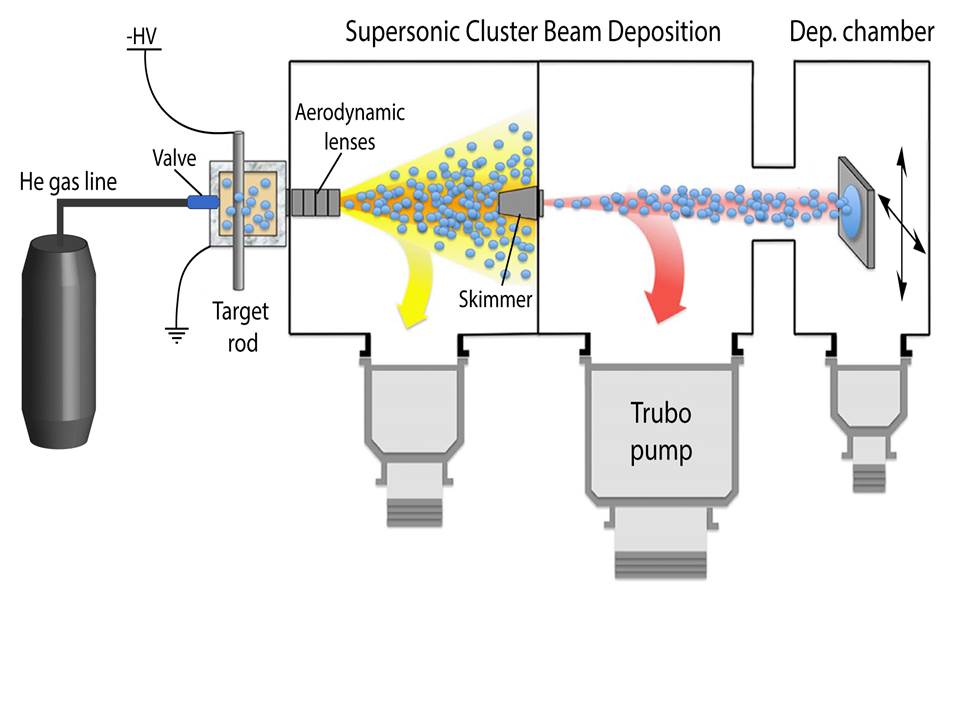
Controlled production of nanoparticles (NPs) is obtained by a gas phase beam source (SCBD), providing flexibility in the material composition, NP tunability, and substrate choice. The following research lines have been developed.
Synthesis of nanomaterials by non-thermal laser ablation (LA)
Chemical Vapor Deposition growth of graphene-based systems
Tailoring nanoparticles by gas phase synthesis
Controlled production of nanoparticles (NPs) is obtained by a gas phase beam source (SCBD), providing flexibility in the material composition, NP tunability, and substrate choice.
The following research lines have been developed.
1.1 Antibacterial coatings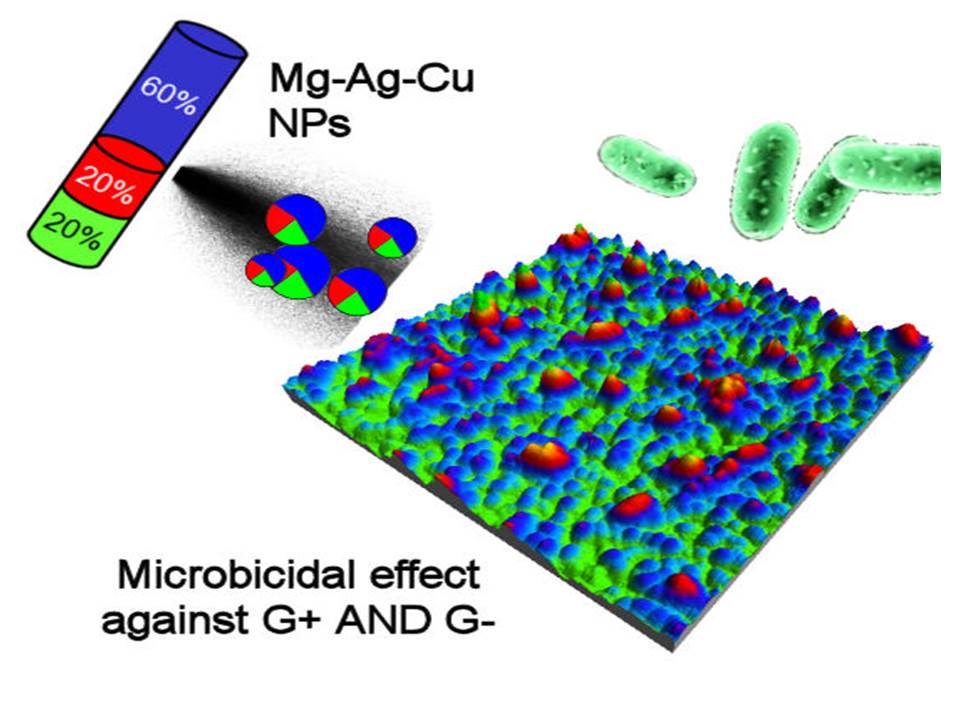
Nosocomial cross contamination through surfaces by antibiotic resistant bacteria is a major global issue. A strategy to decrease the incidence of infections is to develop antibacterial coatings.
The SCBD allows to deposit films of crystalline Ag NPs with narrow size dispersion and density, with high bactericidal activity towards Gram-negative strains. The Ag film is stress free with a Young modulus half of that of standard Ag films and present a good adhesion to sapphire.
1.2 Multi-element NPs
Combining materials into nanogranular structures would open the way to tailor the functionalities of these systems. The SCBD allows to deposit nanogranular structures composed of NP with tunable and mixed chemical composition with two or three different elements within a single NP. The Np have a Janus like or core shell with a crystalline and metallic part embedded in an oxidized and amorphous matrix, that strongly enhances the adhesion to the substrate, and present a very good bactericidal activity. This line will be exploited to study the nanojoining behavior of the granular nanomaterials.
1.3 Photocatalytic properties of NP and mixed fiber-NPs composites
Fibrous Cellulose Acetate nanocomposites filled with SnO2 are doped with metal/semiconductor NPs to degrade organic dyes like MO under ultraviolet irradiation. By combining the SnO2 with Ag nanoparticles on the cellulose acetate fibrous mats, the photocatalytic performance is significantly enhanced compared the Cellulose acetate/SnO2 nanocomposites. Other NPs composites are currently applied to verify the effect on the photocatalytic activity of the fibers.
Nanogranular films composed of TiO2 NPs co-doped with Cr and N have been obtained on different substrates (silicon, LAO, quartz, glass) to respond to the issue of low absorption in the visible range of photocatalytic TiO2. The films have an atomic concentration of substitutional N and Cr of 11 and 5 %, respectively, bringing a substantial decrease of the titanium oxide band gap and strongly enhancing the light absorption in the visible region.
Synthesis of nanomaterials by non-thermal laser ablation (LA)
The LA by high peak intensity femtosecond laser pulses is exploring the synthesis of materials from a ta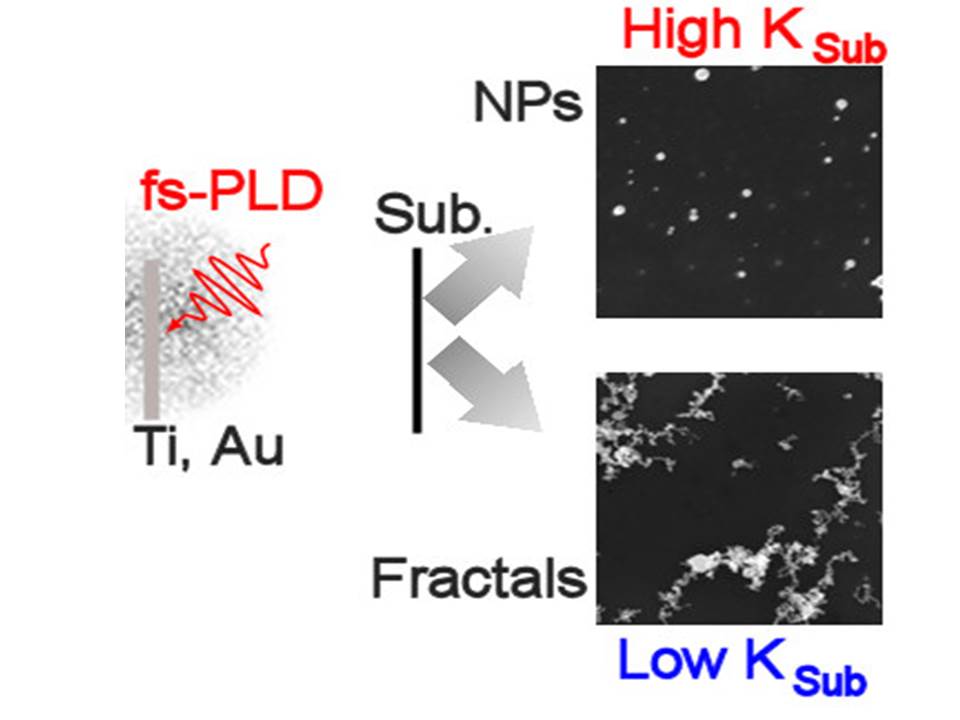 rget where the physics of NP emission does not go through the classical melting neither sublimation processes.
rget where the physics of NP emission does not go through the classical melting neither sublimation processes.
LA deposition at atmospheric pressure is obtained generating a plume of material that is deposited onto a substrate (silicon, graphite, quartz). We obtain fractal TiO2 and Au nanostructures composed by NPs with an average diameter in a range below 20 nm. The fractal dimension and NPs area can be tuned by varying laser fluence and sample/target distance. The fractals are TiO2 with both rutile or anatase phase and pure Au. The comparison of model of NPs diffusion and experiment shows that such fractal aggregates are formed after landing of the ablated material on the substrate surface by a diffusive mechanism. The role of the substrate thermal conductivity is highlighted.
Chemical Vapor Deposition growth of graphene-based systems
Graphene is a one-atom-thick layer of carbon with remarkable electronic properties that have focused the attention of scientists and engineers. In particular, graphene is a semiconductor with zero band gap and high carrier mobilities and concentrations, and shows nearly ballistic transport at room temperature. A challenging aspect of graphene integration into electronic devices and of graphene-based substrates for chemical catalysis is the exfoliation of graphite into individual sheets in a controlled, scalable, and reproducible way. The chemical exfoliation of graphite oxide (using the Hummer oxidation reaction) is efficient, cheap and clean and results in high yields of single-layered graphene oxide (GO). The individual graphene oxide sheets can then be readily deposited on virtually any substrate over large areas using solution based methods. GO forms over a range of O:C stoichio metries, with the oxygen bound to the carbon in the basal plane in the form of hydroxyl and epoxy functional groups and as carbonyl and carboxyl groups at the sheet edges.
metries, with the oxygen bound to the carbon in the basal plane in the form of hydroxyl and epoxy functional groups and as carbonyl and carboxyl groups at the sheet edges.
The oxygen functional groups make graphene oxide sheets strongly hydrophilic and decrease the interaction energy between the graphene layers. Hence, graphite oxide can be readily exfoliated, forming a stable aqueous dispersion.
We are able to study both the changes in the surface morphology of the films (SEM - Scanning Electron Microscopy) and the chemical modifications of the surface after the exposition to the nitrogen plasma (XPS - X-ray Photoelectron Spectroscopy). Finally, the obtained N-doped GO systems is tested as efficient metal-less catalyst for the Oxygen Reduction Reaction (ORR).
